Robert Bunn Office Furniture Specialists

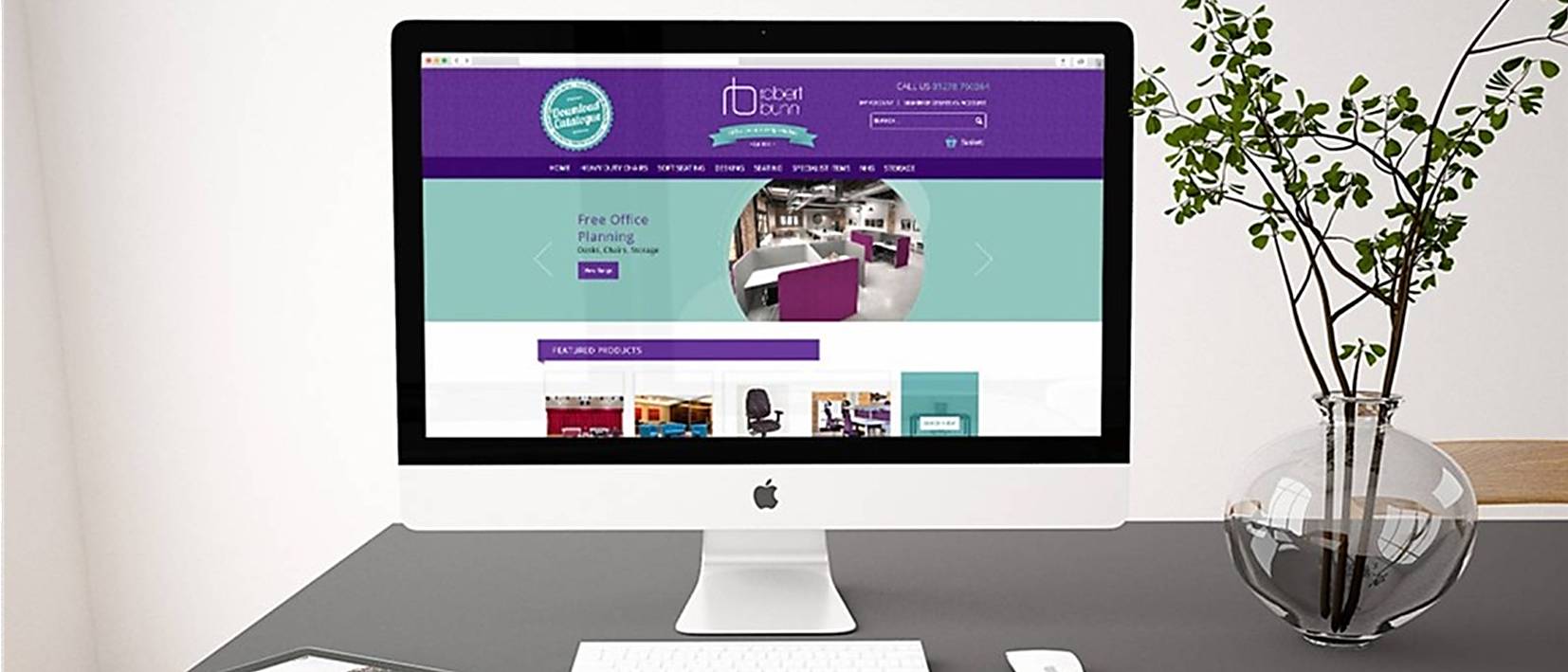

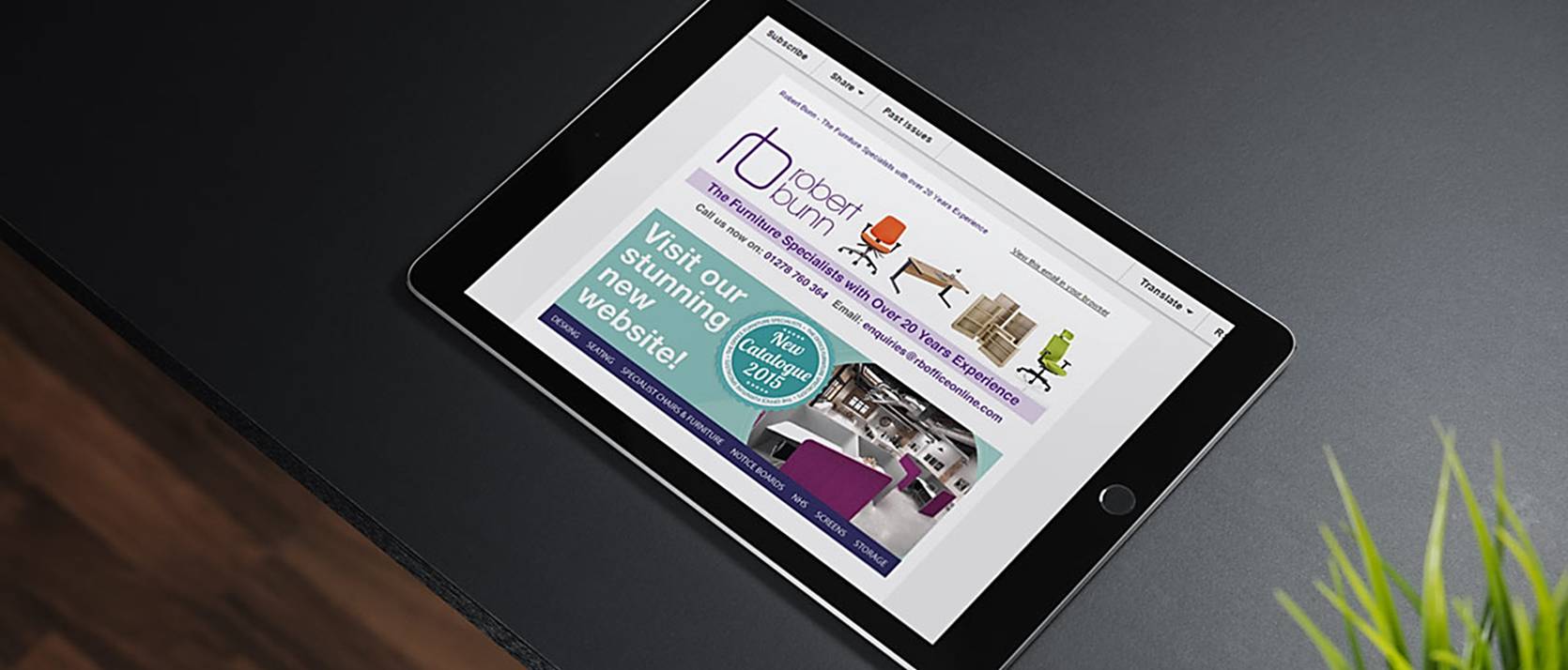

Making the serious business of an ergonomic working environment appealing, easy to navigate and informative within an SEO supported, ecommerce website for increased sales was the brief given to icd from this most prestigious of business retailers.
The solutions included:
- Logo & branding
- Ecommerce website
- News & sales letters – design and copywriting
- Banners
- Advertorials